Scorpio Gold Corp. {TSX.V: SGN} announced its operating results for the first quarter of 2017 at its 70% owned Mineral Ridge project, located in Nevada.
Gold and silver production in Q1 2017 totalled 5,741 ounces and 2,854 ounces, respectively. The lower metal production in Q1 2017 is attributed to fewer tons being mined and processed from the existing pits, due to higher strip ratios that constrain how quickly benches can be mined.
Scorpio Gold Produces 5,741 Ounces of Gold in First Quarter 2017 at the Mineral Ridge Operation, Nevada
Vancouver, April 28 2017 – Scorpio Gold Corp. {TSX.V: SGN} announces its operating results for the first quarter (“Q1”) of 2017 at its 70% owned Mineral Ridge project, located in Nevada.
Gold and silver production in Q1 2017 totalled 5,741 ounces and 2,854 ounces, respectively. The lower metal production in Q1 2017 is attributed to fewer tons being mined and processed from the existing pits, due to higher strip ratios that constrain how quickly benches can be mined.
Brian Lock, interim CEO, reports, “The first quarter production at Mineral Ridge was affected by a slowdown in mining and processing while the project awaits approval of the amendment to its revised Plan of Operations, which is pending from applicable regulatory authorities. The Custer pit and other areas for which permitting is outstanding, will be evaluated for economics of associated mining timelines when permits are received.”
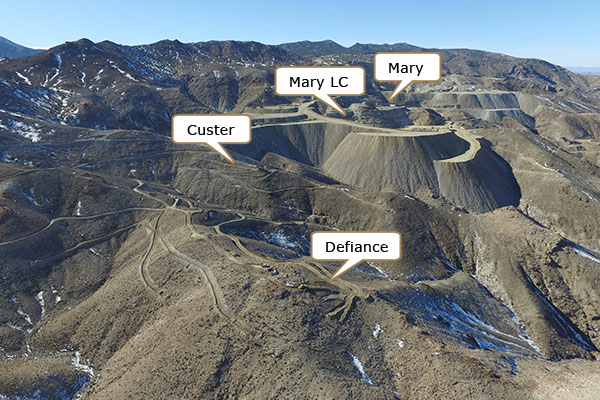
Production in 2017 is scheduled from the Mary LC pit and from the Bluelite and Brodie satellite pits.
Key Operating Statistics
Q1 2017 Q1 2016 %Change
Mining operations
Mary LC pit
Ore tonnes mined 130,446 146,872 -11.2%
Waste tonnes mined 927,786 703,030 32.0%
Total mined 1,058,232 649,902 24.5%
Strip Ratio 7.1 4.8 47.9%
Satellite pits
Ore tonnes mined 7,255 103,252 93.0%
Waste tonnes mined 67,208 227,056 70.4%
Total mined 74,463 330,308 -77.5%
Strip Ratio 9.3 2.2 322.7%
Total producing pits
Ore tonnes mined 137,701 250,124 -44.9%
Waste tonnes mined 994,994 930,086 -4.0%
Total mined 1,132,695 1,180,210 -4.0%
Strip Ratio 7.2 3.7 94.6%
Pits under development
Ore tonnes mined 178 – 100.0%
Waste tonnes mined (pre-stripping) 212,595 55,622 282.2%
Total mined 212,773 55,622 282.5%
Total mining operations
Ore tonnes mined 137,879 250,124 -44.9%
Waste tonnes mined 1,207,589 985,708 22.5%
Total mined 1,345,468 1,235,832 8.9%
Processing
Tonnes processed 138,392 251,587 -45.0%
Gold head grade (g/t) 1.70 1.65 3.0%
Ounces produced
Gold 5,741 8,508 -32.5%
Silver 2,854 3,921 –27.2%
Recoverable(1) gold (ounces) placed on pad 5,175 9,032 -42.7%
(1) A weighted average metallurgical recovery factor has been applied to the estimated contained ounces crushed and placed on the leach pad based on the pit from which the ore was mined.
About Scorpio Gold
Scorpio Gold holds a 70% interest in the Mineral Ridge gold mining operation located in Esmeralda County, Nevada with joint venture partner Elevon, LLC (30%). Mineral Ridge is currently in production as a conventional open pit mining and heap leach operation. The Mineral Ridge property is host to multiple gold-bearing structures, veins and lenses at exploration, development and production stages. Scorpio Gold also holds a 100% interest in the advanced exploration-stage Goldwedge property in Manhattan, Nevada, with a fully permitted underground mine and 400 ton per day mill facility. The Goldwedge mill facility has been placed on a care and maintenance basis and can be restarted on short notice.
Scorpio Gold’s Chairman, Peter J. Hawley, PGeo., is a Qualified Person as defined by National Instrument 43-101 and has reviewed and approved the content of this release.
ON BEHALF OF THE BOARD
SCORPIO GOLD CORPORATION
Brian Lock,
Interim CEO
For further information contact:
Chris Zerga, President
+1 819 825 7618
Website: www.scorpiogold.com
Neither TSX Venture Exchange nor its Regulation Services Provider (as that term is defined in the policies of the TSX Venture Exchange) accepts responsibility for the adequacy or accuracy of this release.
The Company relies on litigation protection for forward-looking statements. This news release contains forward-looking statements that are based on the Company’s current expectations and estimates. Forward-looking statements are frequently characterized by words such as “plan”, “expect”, “project”, “intend”, “believe”, “anticipate”, “estimate”, “suggest”, “indicate” and other similar words or statements that certain events or conditions “may” or “will” occur, and include, without restriction, any statements regarding the Company receiving approval of its pending Plan of Operations amendment. Such forward-looking statements involve known and unknown risks, uncertainties and other factors that could cause actual events or results to differ materially from estimated or anticipated events or results implied or expressed in such forward-looking statements, including risks involved in mineral exploration and development programs, risks involved in mineral processing; risks related to open pit mining and heap leach processing operations, obtaining the required permits to expand and extend mining activities and those risk factors outlined in the Company’s Management Discussion and Analysis as filed on SEDAR. Any forward-looking statement speaks only as of the date on which it is made and, except as may be required by applicable securities laws, the Company disclaims any intent or obligation to update any forward-looking statement, whether as a result of new information, future events or results or otherwise. Forward-looking statements are not guarantees of future performance and accordingly undue reliance should not be put on such statements due to the inherent uncertainty thereof.
Copyright © 2012 SCORPIO GOLD CORPORATION (TSX: SGN) All rights reserved.
For more information please visit our website at http://www.scorpiogold.com/
or send email to scorpio@scorpiogold.com